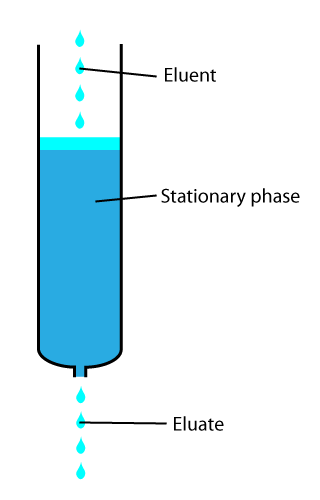
 After cyclic loading molecules onto a HP20 column, the next step is to get them off again in groups of molecules with similar polarity. We do this by passing solvents of different polarity through the column. Molecules on the column with similar polarity to that of the solvent will dissolve into the solvent and be carried out of the column and into a conical flask. This process is called elution. The solvent that passes through the column is the eluent. Once it emerges from the column containing dissolved molecules it is referred to as the eluate.
After cyclic loading molecules onto a HP20 column, the next step is to get them off again in groups of molecules with similar polarity. We do this by passing solvents of different polarity through the column. Molecules on the column with similar polarity to that of the solvent will dissolve into the solvent and be carried out of the column and into a conical flask. This process is called elution. The solvent that passes through the column is the eluent. Once it emerges from the column containing dissolved molecules it is referred to as the eluate.The first solvent used is water. As water is quite polar and there should be few if any significantly polar molecules on the column, very little material should be removed by the water. Its role is really just to rinse the column of any salts or very polar molecules that may have become attached.
The next solvent used is 30% acetone (30% acteone / 70% water by volume). This solvent is less polar than water and will remove moderately polar molecules from the column. The eluate is collected in a conical flask.
Then it is the turn of 75% acetone (75% acetone / 25% water by volume). This removes a group of less polar molecules.
Finally 100% acetone gets passed through the column and removes molecules of very low polarity (and non-polar molecules).
We refer to the solutions gained as the 30% fraction, 75% fraction and 100% fraction. Each fraction will contain a range of molecules that still need to undergo further sorting.
No comments:
Post a Comment